Dissolved Gases
Dissolved gases are considered to be impurities and these are of two types wiz Non-reactive gases and Reactive gases.
Non-reactive Gases:
Nonreactive gases are dissolved gases, such as oxygen and nitrogen, that do not react with water to produce ionic contaminants to the solution and affect the pH of the water. The solubility of both oxygen and nitrogen in water at a constant pressure is a function of temperature, with solubility decreasing with increasing temperature. The presence of dissolved nonreactive gases in water does not have any significant effect on pharmaceutical water purification system performance. The presence of oxygen in product water using stainless steel storage and distribution systems may oxidize stainless steel surfaces. However, with very minor exceptions, the removal of dissolved nonreactive gases in pharmaceutical water purification systems is rarely used.
Reactive Gases:
Single-pass and double-pass RO systems extensively used for the production of USP Purified Water, some active pharmaceutical ingredient (API) manufacturing operations, and for pre-treatment of feed water to Water for Injection (WFI) systems. Presence of dissolved reactive gases can have a intense impact on the quality of product water. The primary dissolved reactive gases of anxiety are ammonia (NH3) and carbon di-oxide (CO2). These gases have significant effect on treatment process so that, it is good practice to provide/ get a comprehensive explanation of the source, anticipated concentration, and effect on pH of both gases.
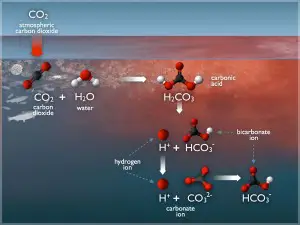
Carbon dioxide is present in air and readily dissolves in water. Its solubility as a function of temperature. As carbon dioxide gas is reactive, it undergoes equilibrium chemical reactions with water as follows:
CO2 + H2O ↔ H3O+ + HCO3–
HCO3– + H2O ↔ H3O+ + CO32-
The primary reaction encountered at anticipated pH values of raw water is the first equation. Obviously, equilibrium is affected by pH, the lower the pH value, the greater the hydronium ion concentration. As the hydronium ion concentration increases (pH decreases), the equilibrium reaction is forced to the left, producing more carbon dioxide and less bicarbonate ion (HCO3–). Conversely, as the pH increases, the hydronium ion concentration decreases. Excess hydroxide ions, associated with the higher pH, will react with hydronium ions, producing water by the following reaction:
H3O+ + OH– ↔ 2H2O
As hydronium ions are removed, the equilibrium reaction is forced to the right, resulting in a lower concentration of carbon dioxide and higher concentration of bicarbonate ions. When ion exchange is used as the ion removal technique within a pharmaceutical water purification system, the equilibrium associated with carbon dioxide has minimal effect on the quality of the product water, since bicarbonate ions are rapidly and continuously removed during passage through anion resin, forcing the equilibrium reaction to the right and eliminating carbon dioxide by removing the bicarbonate ions. On the other hand, single pass or double pass RO systems will allow carbon dioxide to pass directly through the RO membrane as a gas. The product water carbon dioxide, which will re-establish equilibrium with bicarbonate and hydronium ions, affects the purity of the product water by increasing its conductivity.
Ammonia gas is generally present at much lower concentrations than carbon dioxide in raw water. Ammonia is in the form of chloramines, which are produced by the reaction of chlorine and ammonia. Relatively high concentrations of residual chloramines, which are required to obtain the same or similar disinfection properties as chlorine, can be associated with the production of ammonia by the following equation:
2NH2Cl ↔ NHCl2 + NH3
Ammonia is extremely soluble in water and will react with water to produce the ammonium (NH4+) and hydroxide ions as follows:
NH3 + H2O ↔ NH4+ + OH–
Equilibrium is affected by pH. As the pH decreases, hydroxide ions react with hydronium ions, forcing the equilibrium reaction to the right, thus decreasing the concentration of ammonia gas and increasing the concentration of ammonium ion. As the pH increases, the concentration of hydroxide ions increases, forcing the equilibrium reaction to the left, thus increasing the ammonia gas concentration and decreasing the ammonium ion concentration. Ammonia gas passes directly through an RO membrane, decreasing product water conductivity. In ion exchange systems, ammonia gas is entirely eliminated as the ammonium ion passes through cation resin and the equilibrium equation is forced to the right.
Carbon dioxide and ammonia are the two primary reactive gases of concern for pharmaceutical water purification systems. Considering the increased use of single pass and double pass RO units and the USP conductivity specification for Purified Water and Water for Injection, the concentration of carbon dioxide and ammonia is an important parameter.